Certara GlobalSubmit eCTD software Overview
Globalsubmit provides eCTD submission software to mitigate rejection risks in a complex regulatory environment. Differences in regulatory requirements complicate the submission process.
Use Cases
Customers recommend Sending & Publishing Communications, Workflow Management, Onboarding, as the business use cases that they have been most satisfied with while using Certara GlobalSubmit eCTD software.
Business Priorities
Scale Best Practices and Launch New Products are the most popular business priorities that customers and associates have achieved using Certara GlobalSubmit eCTD software.
Certara GlobalSubmit eCTD software Use-Cases and Business Priorities: Customer Satisfaction Data
Certara GlobalSubmit eCTD software's features include Templates. and Certara GlobalSubmit eCTD software support capabilities include 24/7 Support, Email Support, Chat Support, etc. also Certara GlobalSubmit eCTD software analytics capabilities include Custom Reports, and Analytics.
Reviews
"...GlobalSubmits approach to document management is a familiar one intuitive interface, easy implementation that appeals to the novice user, built-in templates and workflows, and integration with eCTD publishing systems...." Peer review
Popular Business Setting
for Certara GlobalSubmit eCTD software
Top Industries
- Pharmaceuticals
Popular in
- Enterprise
Certara GlobalSubmit eCTD software is popular in Pharmaceuticals, and is widely used by Enterprise,
Certara GlobalSubmit eCTD software Customer wins, Customer success stories, Case studies
How can Certara GlobalSubmit eCTD software optimize your Sending & Publishing Communications Workflow?
What benefits does Certara GlobalSubmit eCTD software offer for Workflow Management?
What solutions does Certara GlobalSubmit eCTD software provide for Onboarding?
What benefits does Certara GlobalSubmit eCTD software offer for Content Management?
11 buyers and buying teams have used Cuspera to assess how well Certara GlobalSubmit eCTD software solved their business needs. Cuspera uses 376 insights from these buyers along with peer reviews, customer case studies, testimonials, expert blogs and vendor provided installation data to help you assess the fit for your specific business needs.
Certara GlobalSubmit eCTD software Features
- Low
- Medium
- High
| FEATURE | RATINGS AND REVIEWS |
|---|---|
| Custom Reports | Read Reviews (48) |
| Analytics | Read Reviews (1) |
| CAPABILITIES | RATINGS AND REVIEWS |
|---|---|
| Custom Reports | Read Reviews (48) |
| Analytics | Read Reviews (1) |
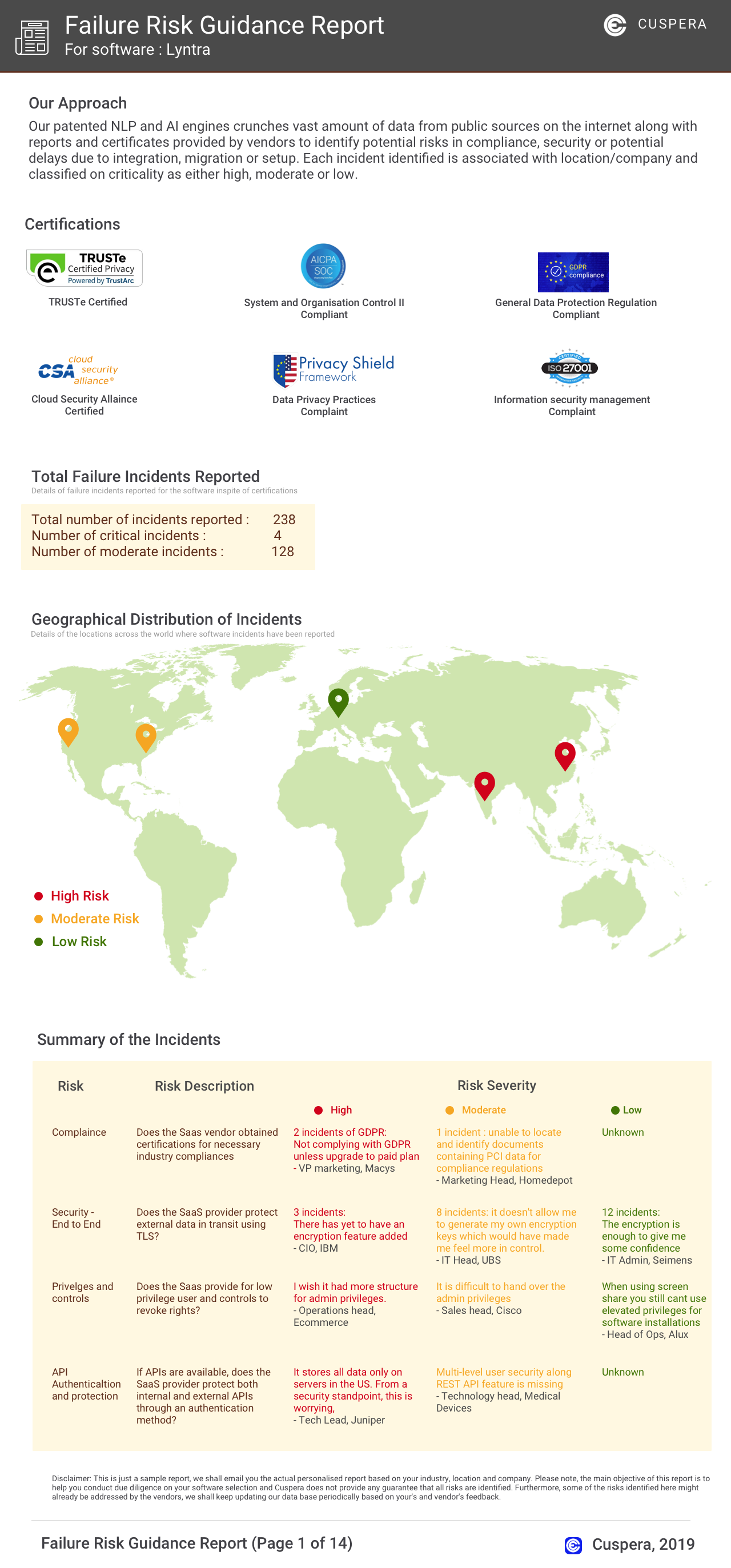
Software Failure Risk Guidance
?for Certara GlobalSubmit eCTD software
Overall Risk Meter
Top Failure Risks for Certara GlobalSubmit eCTD software
Certara, USA. News
Certara Partners with Veeva AI to Automate Regulatory Submissions
Certara has partnered with Veeva AI to integrate Certaras CoAuthor generative AI solution with Veeva RIM, enhancing regulatory submissions for life sciences. This collaboration automates document creation, streamlines data flows, and improves collaboration between data providers and regulatory writers, reducing preparation time by 30%. The integration allows seamless access to source files, expediting submission processes and improving accuracy.
Certara, Inc. to Release Third Quarter 2025 Financial Results on ...
Certara, Inc. to Release Third Quarter 2025 Financial Results on ...
Certara Launches DIDB Concomitant Meds Navigator
Certara has launched the DIDB Concomitant Meds Navigator, a new software tool designed to identify contraindicated concomitant medications during drug studies. This tool, part of Certara's Drug Interaction Database, helps research teams make informed decisions to protect study participants. It extends Certara's leadership in drug interaction science by providing comprehensive, up-to-date data on drug interactions, including investigational drugs and natural products.
Certara to Report Second Quarter 2025 Financial Results on August 6th, 2025 - TradingView
Certara will announce its second quarter 2025 financial results on August 6th, 2025, after market close. A conference call to discuss the results will follow at 5:00 PM ET.
Certara, USA. Profile
Company Name
Certara, USA.
Company Website
https://www.certara.com/HQ Location
123 South Broad Street, Suite 1850, Philadelphia, PA 19109, US
Employees
11-50
Social
Financials
PRIVATE