Ennov Document Overview
Ennov provides a unified content and information management platform tailored for the life sciences sector, addressing the complex challenges of product development. It integrates various functions such as Quality, Clinical, Regulatory, Pharmacovigilance, and Commercial into a single platform. This consolidation facilitates streamlined document management, regulatory compliance, and data analytics, enhancing operational efficiency across the product lifecycle. Ennov's platform supports AI capabilities, enabling smarter and more secure workflows. With over 25 years of experience, Ennov offers flexible deployment options and a comprehensive suite of tools designed to meet the unique needs of life sciences organizations, ensuring compliance and fostering innovation.
Use Cases
Customers recommend Content Management, Helpdesk Management, Competitive Intelligence, as the business use cases that they have been most satisfied with while using Ennov Document.
Business Priorities
Scale Best Practices and Establish Thought Leadership are the most popular business priorities that customers and associates have achieved using Ennov Document.
Ennov Document Use-Cases and Business Priorities: Customer Satisfaction Data
Ennov Document works with different mediums / channels such as Offline. Point Of Sale. and On Premises.
Ennov Document's features include Dashboard, and Personalization. and Ennov Document support capabilities include 24/7 Support, AI Powered, Email Support, etc. also Ennov Document analytics capabilities include Custom Reports, and Analytics.
Reviews
"...The ENNOV team reactivity (support desk but also project manager)..." Peer review by Cyrille J., Regulatory Affairs Systems Manager
Ennov Doc for Quality is a comprehensive solution to manage, share, and track documentation per GxP and ISO standards. Documentation is efficiently managed and standards compliance ensured.
Popular Business Setting
for Ennov Document
Top Industries
- Mechanical or Industrial Engineering
Popular in
Ennov Document is popular in Mechanical Or Industrial Engineering, and is widely used by
Ennov Document Customer wins, Customer success stories, Case studies
How does Ennov Document address your Content Management Challenges?
What solutions does Ennov Document provide for Helpdesk Management?
What benefits does Ennov Document offer for Competitive Intelligence?
How can Ennov Document enhance your Workflow Management process?
Why is Ennov Document the best choice for Sending & Publishing Communications?
11 buyers and buying teams have used Cuspera to assess how well Ennov Document solved their business needs. Cuspera uses 306 insights from these buyers along with peer reviews, customer case studies, testimonials, expert blogs and vendor provided installation data to help you assess the fit for your specific business needs.
Ennov Document Competitors
Ennov Document Features
- Low
- Medium
- High
| FEATURE | RATINGS AND REVIEWS |
|---|---|
| Custom Reports | Read Reviews (44) |
| Analytics | Read Reviews (17) |
| CAPABILITIES | RATINGS AND REVIEWS |
|---|---|
| Custom Reports | Read Reviews (44) |
| Analytics | Read Reviews (17) |
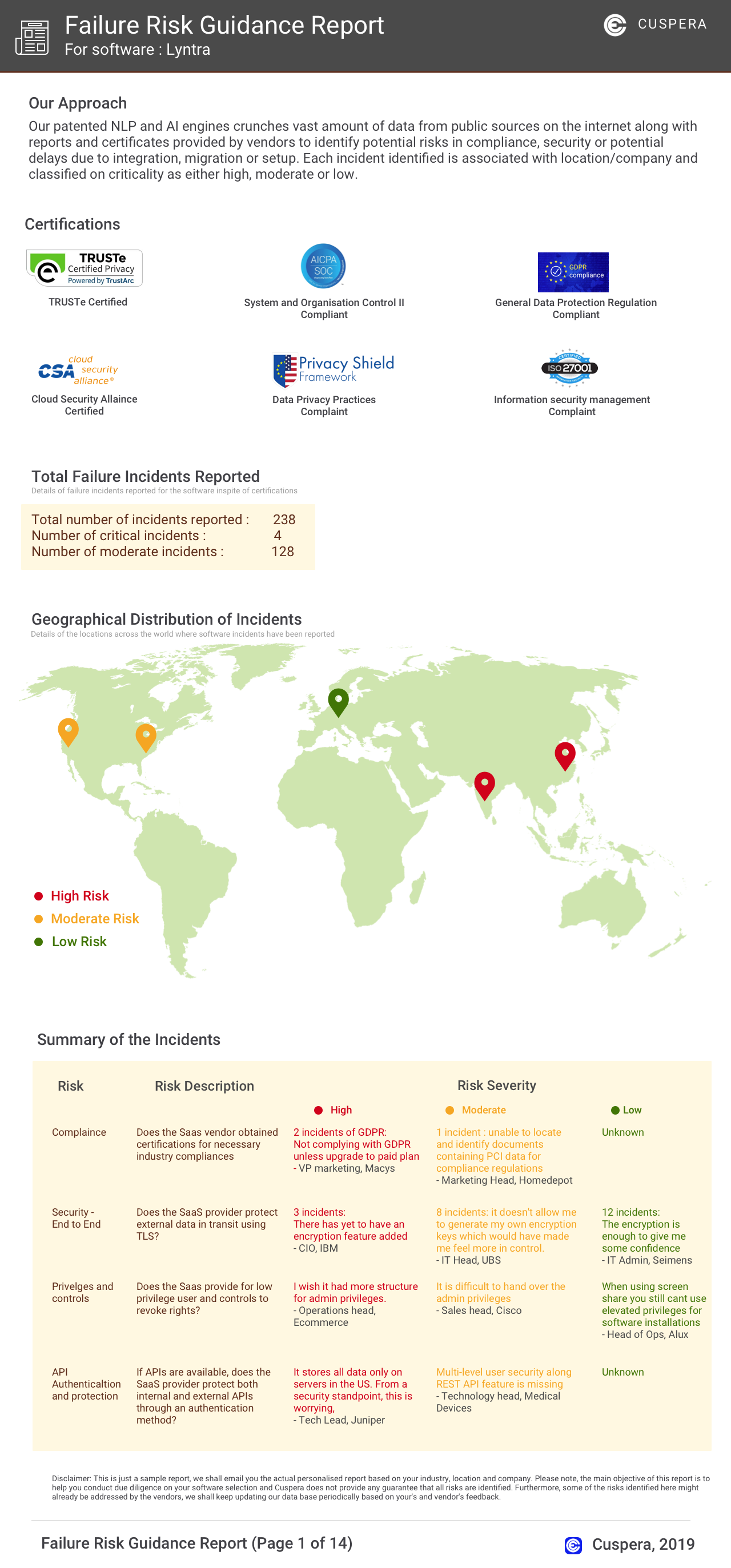
Software Failure Risk Guidance
?for Ennov Document
Overall Risk Meter
Top Failure Risks for Ennov Document
Ennov SAS News
Ennov SAS Profile
Company Name
Ennov SAS
Company Website
https://en.ennov.com/HQ Location
Greensboro, North Carolina US
Employees
101-250
Social
Financials
PRIVATE